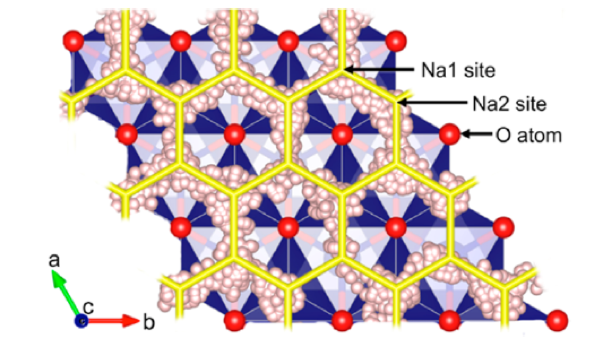
Figure: Ab initio molecular dynamics study demonstrating Na-ion diffusion mechanism in P2-type layered transition metal oxides used as Na-ion battery cathodes.
Sodium-ion batteries have vast potential as an inexpensive, geopolitically-neutral alternative to Li-based rechargeable batteries, largely due to the global abundance and low cost of sodium-containing precursor materials. Na-ion electrode materials, while conceptually similar to their Li-ion counterparts, give rise to unique challenges and physical properties due to sodium’s larger size and lower absolute standard electrode potential. Due to their larger size, Na ions can reversibly intercalate in more materials than Li ions, giving a much broader chemical space in which electrode materials can be optimized. This, together with sodium’s abundance, makes Na-ion batteries excellent candidates for large-scale energy storage.
The Ceder Group conducts computational and experimental studies with the goal of novel Na-ion electrode and electrolyte discovery, as well as material optimization and understanding of the underlying physics governing material function. First-principle computational efforts are used to obtain insights into phase transitions during electrochemical deintercalation of Na-ion cathodes, as well as diffusion mechanics and diffusivity. High throughput computation is utilized to search for novel cathode, anode, and solid electrolyte materials. Our experimental studies give rise to novel electrode synthesis and testing, electrolyte optimization, and insights into the influence of transition metals and Na-ion superstructure formation on Na-ion battery function.
Our combined theoretical and computational efforts have led to the synthesis of state-of-the-art layered oxide Na-ion cathodes with excellent rate capabilities and the highest energy densities currently found in literature. As part of these efforts, we have also successfully achieved directly visualization of the Jahn-Teller coupled to Na-ion ordering in layered transition metal oxides.
Select Publications:
- Toumar, A., Ong, S. P. Richards, W., Dacek, S., Ceder, G., Vacancy ordering in layered metal oxide Na-ion battery cathodes. Rev. Applied, 4, 064002(2015). http://journals.aps.org/prapplied/abstract/10.1103/PhysRevApplied.4.064002.
- Matts, I., Dacek, S., Pietrzak, T. K., Malik, R., Ceder, G., Explaining performance-limiting mechanisms is fluorophosphate Na-ion battery cathodes through inactive transition-metal mixing and first-principles mobility calculations, Chemistry of Materials, 27(17), 6008-6015(2015). http://pubs.acs.org/doi/full/10.1021/acs.chemmater.5b02299.
- Liu, L., et al, High Performance P2-type Na2/3(Mn1/2Fe1/4Co1/4)O2 Cathode Material with Superior Rate Capability for Na-ion Batteries, Advanced Energy Materials 5, 1500944(2015)
- Li, X., et al, O3-Type Na(Mn25Fe0.25Co0.25Ni0.25)O2: A quaternary layered cathode compound for rechargeable Na ion batteries, Electrochem. Comm.,49, 51(2014). http://onlinelibrary.wiley.com/doi/10.1002/aenm.201500944/abstract.
- Li, X., et al, Direct visualization of the Jahn–Teller effect coupled to Na ordering in Na5/8MnO2, Nature Mater, 13(6), 586-592(2014). http://www.nature.com/nmat/journal/v13/n6/full/nmat3964.html.
- Ong, S. P., Chevrier, V. L., Hautier, G., Jain, A., Moore, C., Kim, S., Ma, X., et al., Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy & Environmental Science, 4(9), 3680–3688(2011). http://pubs.rsc.org/en/Content/ArticleLanding/2011/EE/c1ee01782a#!divAbstract.