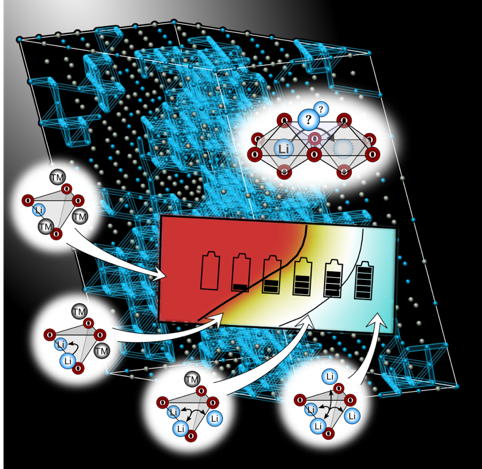
Figure: Large-scale Monte-Carlo simulations of lithium percolation based on the atomic-scale lithium diffusion mechanism allow the prediction of macroscopic lithium transport properties [2,3]. The output of such simulations, percolation maps such as the one shown in the figure, provide a practical guideline for the development of new cathode materials [4,5]. The background of the figure shows the percolating network of fast lithium diffusion channels in a cation-disordered rocksalt-type oxide.
Over the past two decades, Li-ion batteries have enabled society-changing technological advances, especially in portable electronic devices such as smart phones and tablet computers and in electric vehicles. To keep up with the ever-increasing demand for energy storage, an enormous worldwide research effort has been geared towards the discovery of new, safe Li-ion battery materials with greater energy densities.
The Ceder group has contributed to this quest by developing robust computational approaches for the prediction of voltage profiles, thermal stabilities, ionic conductivities, and Li percolation, to name just a few examples [1]. Taking the insight from electronic structure calculations into the lab, the Ceder group has been at the forefront of rational materials design. Some examples where computational understanding enabled breakthrough discoveries of new Li-ion battery cathode materials are the design of rate-enhanced Li(Ni0.5Mn0.5)O2 [6], the realization of high-rate lithium iron phosphate [7], and the discovery of high-capacity cation-disordered oxides [2].
Select Publications:
- A. Urban, D.-H. Seo, G. Ceder, Computational understanding of Li-ion batteries, npj Computational Materials 2 16002; doi:10.1038/npjcompumats.2016.2; (2016). http://www.nature.com/articles/npjcompumats20162
- J. Lee, A. Urban, X. Li, D. Su, G. Hautier, G. Ceder, Unlocking the Potential of Cathode-Disordered Oxides for Rechargeable Batteries, Science, Science 343 (2014) 519-522. http://science.sciencemag.org/content/343/6170/519.full.pdf+html
- A. Urban, J. Lee, G. Ceder, The Configurational Space of Rocksalt-Type Oxides for High-Capacity Lithium Battery Electrodes, Adv. Energy Mater. 2014, 4, 1400478, DOI: 10.1002/aenm.201400478, http://onlinelibrary.wiley.com/doi/10.1002/aenm.201400478/full.
- N. Twu, X. Li, A. Urban, M. Balasubramanian, J. Lee, L. Liu, G. Ceder, Designing New Lithium-Excess Cathode Materials from Percolation Theory: Nanohighways in LixNi2-4x/3Sbx/3O2, Nano Lett., 2015, 15 (1), pp 596–602. http://pubs.acs.org/doi/full/10.1021/nl5040754?mobileUi=0.
- Y. Wang, W.D. Richards, S.P. Ong, L.J. Miara, J.C. Kim, Y. Mo, G. Ceder, Design principles for solid-state lithium superionic conductors, Nature Materials14,1026–1031doi:10.1038/nmat4369, http://www.nature.com/nmat/journal/v14/n10/abs/nmat4369.html.
- K. Kang, Y.S. Meng, J. Bréger, C.P. Grey, and G. Ceder, Electrodes with high power and high capacity for rechargeable lithium batteries, Science 311 (2006) 977-980. http://science.sciencemag.org/content/311/5763/977.
- K. Kang and G. Ceder, Battery materials for ultrafast charging and discharging, Nature 458 (2009) 190-193. http://www.nature.com/nature/journal/v458/n7235/abs/nature07853.html.