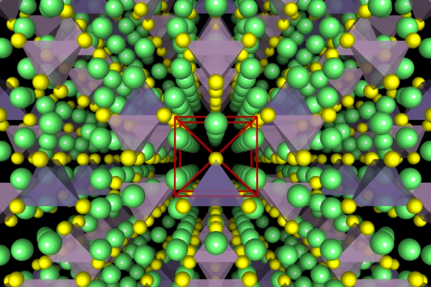
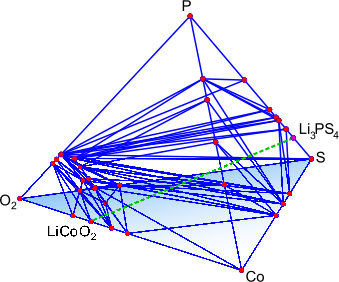
Figure: (left) Crystal structure of the superionic conductor Li10GeP2S12. The backbone of the material is a body-centered cubic-like arrangement of sulfur anions, (right) DFT-computed phase diagram for reaction products at a Li3PS4-LiCoO2 solid-state-battery interface.
Safety issues are an immense concern in developing advanced energy storage technologies, especially for Li-ion batteries, which contain flammable organic liquid electrolyte. Replacing organic liquid electrolytes with a solid-state ionic conductor would improve battery safety and remove one of the few remaining barriers to even wider-scale use of Li-ion technology. Inorganic solid-state Li-ion conductors also benefit from many other advantages such as superior electrochemical, mechanical and thermal stability, absence of leakage, and the possibility of battery miniaturization.
The Ceder group has been developing and using advanced first-principles computational methodologies, in combination with a materials genome approach, to search for and optimize novel Li-ion superionic conductors with superior ionic conductivity and chemical and electrochemical stability. To study ionic diffusion in solid-state conductors, we have established high-throughput computational methods based on ab initio molecular dynamics to obtain conductivities and activation energies even in highly complex crystalline structures. We also complement these AIMD simulations with nudged elastic band (NEB) simulations, allowing higher-resolution investigation into the structural and chemical determinants of ionic diffusion. Very recently, we have utilized an accurate and efficient thermodynamic methodology to evaluate chemical and electrochemical stabilities and to explain trends in observed experimental phenomena for specific electrolyte/electrode interfaces. Our group was the first to propose modifications of the superionic conductor Li10GeP2S12 (LGPS) and to predict its very limited stability window [1,2].
Select Publications:
- S.P. Ong, Y. Mo, W.D. Richards, L. Miara, H.S. Lee, G. Ceder, Phase stability, electrochemical stability and ionic conductivity of the Li10+/-1MP2X12 (M = Ge, Si, Sn, Al or P, and X = 0, S or Se) family of superionic conductors, Energy Environ. Sci., 2013, 6, 148-156, DOI: 10.1039/C2EE23355J, http://pubs.rsc.org/en/content/articlehtml/2013/ee/c2ee23355j.
- Y. Mo, S.P. Ong, G. Ceder, First Principles Study of the Li10GeP2S12 Lithium Super Ionic Conductor Material, Chem. Mater., 2012, 24 (1), pp 15–17, DOI: 10.1021/cm203303y, http://pubs.acs.org/doi/abs/10.1021/cm203303y.
- Wang, W. D. Richards, S. P. Ong, L. J. Miara, J. C. Kim, Y. Mo, and G. Ceder. Design Principles for Lithium Superionic Conductors. Nat. Mater. 14, 1026–1031 (2015). http://www.nature.com/nmat/journal/v14/n10/full/nmat4369.html.
- D. Richards, L. J. Miara, Y. Wang, J. C. Kim, G. Ceder. Interface Stability in Solid-State Batteries, Chem. Mater 28, 266-273 (2016). http://pubs.acs.org/doi/full/10.1021/acs.chemmater.5b04082.